Although the primary purpose of polypharmacy reviews is in deriving clinical benefits, they also deliver long-term direct and indirect economic benefits. A direct reduction in the cost of prescribing, and reduction in medicines waste is anticipated. In terms of indirect economic benefits, a patient stabilised on fewer medicines will likely require less contact with health professionals, thereby freeing up capacity. Of prime aim is the indirect economic benefit of fewer unscheduled hospital admissions due to adverse drug reactions (ADRs).
Appendix D: Health Economics Analysis of Polypharmacy Reviews
The goal of the SIMPATHY Economic Analysis tool 66, developed as part of the EC SIMPATHY project, was to provide a high-level analysis of the economic costs and benefits associated with carrying out polypharmacy reviews. The analysis follows a top-down approach and estimates maximum costs and benefits associated with activity. Activity is driven by the selected population for whom reviews are intended to be carried out.
Costs of reviews are based on the resource (staff) cost of carrying out a review, net of any potential review charge. The direct potential financial benefit of reviews will consist of the net reduction in drugs prescribed, and associated expenditure. Potential indirect benefits (non-cash releasing) centre around potentially avoided Adverse Drug Reactions (ADRs), preventable hospital admissions associated with these ADRs, and the associated number of hospital bed days avoided. The costs of medicines stopped and reduced are cash releasing, whereas avoided admissions are a capacity release productive opportunity.
Ultimately, the tool was intended to add to the package of SIMPATHY change management tools by offering a bespoke analysis of the micro-economic impacts, the costs and benefits of introducing and carrying out reviews. It is thought that this will give a broad overview around resource needs and potential benefits to interested users.
Structure of the SIMPATHY model
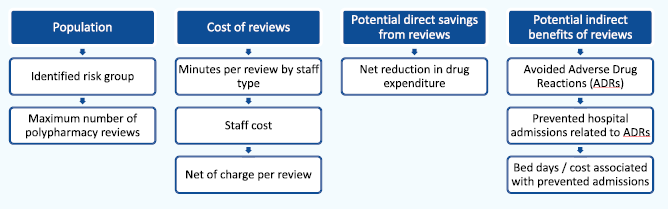
Table D1 (below) provides an overview of estimated activity and associated costs per review for Scotland. A range of different models and estimates are provided with some variation in the way that this information was provided. Renewed estimates range from £24.36 to just over £67 per review, which is a reduction on earlier work. It should also be noted that these cost estimates are a monetisation of assumed core clinical activity, and will therefore not pose an additional cost.
Net reductions in the number of items stopped over one year were estimated to be in a range of between 4.9 and 18.2 items, and an averageof 11.9 items (number of reviews per annum, applied to the net of the number of drugs stopped/decreased minus those started/increased, and their average number of repeats). That range is then applied to a lower and an upper estimate of costs per item (£10.17 and £10.90)A to give a full range of the potential direct savings from net reductions in drugs, ranging from £50 to £200.
AItem cost estimates are quarter 3, 2016/17 only, to acknowledge more accurately the current cost of prescriptions, but not taking seasonality into consideration. Includes items prescribed on GP10 forms only, excludes prescribed by pharmacists, nurses, etc, to avoid inclusion of stock orders and medicines supplied from hospital and CPU forms. Excludes appliances and vaccines as these are not therapeutic treatments considered in polypharmacy reviews
Lower estimate includes BNF chapters: 01;02;03;04;05;06;07;09;10;11;12
Upper estimate includes all BNF chapters
Pirmohamed (2004) estimate a prevalence of 6.5% (95% C.I. 6.2% to 6.9%) of admissions judged as being due to an ADR. The study determined avoidability of admissions related to an ADR. Only 28% (25% to 30%) of the ADRs were assessed as unavoidable, while 9% (7% to 10%) were classified as definitely avoidable and 63% (60% to 66%) as possibly avoidable.
Applying these parameters, and an additional conservative assumption that 10% of avoided admissions (and associated bed days) are avoided due to polypharmacy reviews, to a population of 1,000 gives the associated indirect benefits presented in Table D4 (below, central estimates only). Note that this also gives a variation in results depending on different types of population groups, each stratified by their level of risk of admission or readmission via Scottish Patients at Risk of Readmission and Admission (SPARRA) database.
Tables 1a and 1b in Appendix G summarise SPARRA population groups. Applying the estimated ranges of costs, and direct and indirect benefits (central estimates) to the population of, e.g. the 75+ SPARRA group (and underlying admissions data) generates the set of results summarised in Table D3 (below).B
BCost and benefit are per annum, given the assumption that these are derived as a follow on from the first review
Table D4 (below) shows the net benefit of deducting the range of costs from savings from all benefits. If all indirect benefits are taken into account, the net benefit is positive throughout. Note that, in the most pessimistic scenario with maximum costs and minimum drug savings, the balance is tipped and can become negative if only direct benefits are taken into consideration.
|
Different models of review staff time allocation |
Staff type |
AfC Band (where appropriate) |
Preparation (work-up) |
Face to Face review |
Follow-up and Related activities1 |
Total time taken |
Total cost per review2 |
|||||
|
min |
max |
min |
max |
min |
max |
min |
max |
min |
max |
|||
|
Type |
Band |
minutes |
minutes |
minutes |
minutes |
minutes |
minutes |
minutes |
minutes |
£ |
£ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2015 guidance |
Clinical Pharmacist |
8a |
|
|
60 |
60 |
15 |
15 |
75 |
75 |
£40.61 |
£40.61 |
|
|
GP |
n/a |
|
|
15 |
15 |
15 |
15 |
30 |
30 |
£26.40 |
£26.40 |
|
|
Total cost |
|
|
|
|
|
|
|
|
|
£67.01 |
£67.01 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Highland |
|
|
|
|
|
|
|
|||||
|
Model 1 - First review |
Clinical Pharmacist |
8a |
5 |
5 |
15 |
15 |
40 |
40 |
60 |
60 |
£32.48 |
£32.48 |
|
|
Total cost |
|
|
|
|
|
|
|
|
|
£32.48 |
£32.48 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Model 2 - Follow-up |
Clinical Pharmacist |
8a |
5 |
5 |
10 |
10 |
35 |
35 |
50 |
50 |
£27.07 |
£27.07 |
|
review |
Total cost |
|
|
|
|
|
|
|
|
|
£27.07 |
£27.07 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tayside |
|
|
|
|
|
|
|
|
|
|
|
|
|
Model 1 - independent |
Clinical Pharmacist |
8a |
15 |
30 |
30 |
30 |
|
|
45 |
60 |
£24.36 |
£32.48 |
|
Pharm prescriber |
Total cost |
|
|
|
|
|
|
|
|
|
£24.36 |
£32.48 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Model 2 - non |
Clinical Pharmacist |
7 |
15 |
30 |
|
|
15 |
30 |
30 |
60 |
£14.15 |
£28.30 |
|
-independent prescriber, |
GP |
n/a |
|
|
|
|
15 |
15 |
15 |
15 |
£13.20 |
£13.20 |
|
With GP review |
Total cost |
|
|
|
|
|
|
|
|
|
£27.35 |
£41.51 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Model 3 - consultant |
GP |
n/a |
|
|
|
|
15 |
15 |
15 |
15 |
£13.20 |
£13.20 |
|
clinic, with GP follow-up |
Geriatric consultant |
n/a |
|
|
30 |
30 |
|
|
30 |
30 |
£42.00 |
£42.00 |
|
|
Total cost |
|
|
|
|
|
|
|
|
|
£55.20 |
£55.20 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ayrshire and Arran3 |
Clinical Pharmacist |
8a |
|
|
|
|
|
|
80 |
120 |
£43.31 |
£64.97 |
|
|
Total cost |
|
|
|
|
|
|
|
|
|
£43.31 |
£64.97 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GG&C4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Model 1 - non |
Clinical Pharmacist |
7 |
30 |
30 |
30 |
30 |
|
|
60 |
60 |
£28.30 |
£28.30 |
|
-independent prescriber, |
GP |
n/a |
|
|
|
|
5 |
10 |
5 |
10 |
£4.40 |
£8.80 |
|
with GP review |
Total cost |
|
|
|
|
|
|
|
|
|
£32.70 |
£37.10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Model 2 - independent |
Clinical Pharmacist |
8a |
10 |
30 |
30 |
30 |
|
|
40 |
60 |
£21.66 |
£32.48 |
|
pharm. prescriber, |
Pharmacy tech. |
5 |
15 |
5 |
|
|
|
|
15 |
5 |
£5.26 |
£1.75 |
|
With tech. support |
Total cost |
|
|
|
|
|
|
|
|
|
£26.91 |
£34.24 |
1 Follow-up and related activities include: Follow-up; MDT meetings; practice meetings; travel; other activities
2 Estimated Weighted Total Cost including on-cost, AfC 2015-16
3 based on Advisers carrying out 2-3 reviews during half-day sessions (4hrs)
4 models for AfC band 7 and band 8a led reviews. Local variation around tech support, less tech support requires more pharmacist preparation time
|
Population = 1,000 |
No risk strati- fication |
Risk stratification |
|||
|
BNF10+ |
BNF10+ & High Risk Med |
BNF 5-9 |
BNF 5-9 & High Risk Med |
||
|
Definitely avoidable hospital bed days* |
0.9 |
8.4 |
7.3 |
7.6 |
6.6 |
|
Assoc. cost avoidance of definitely avoidable admissions |
£326 |
£3,110 |
£2,699 |
£2,801 |
£2,421 |
|
Possibly avoidable hospital bed days |
6.2 |
59.1 |
51.3 |
53.2 |
46.0 |
|
Assoc. cost avoidance of possibly avoidable admissions |
£2,280 |
£21,771 |
£18,891 |
£19,604 |
£16,945 |
|
* Including assumption that 10% of avoided bed days are avoided due to polypharmacy reviews |
|
|
|||
|
Total in group |
42,882 |
|
|
|
||
|
Direct costs and benefits |
minimum |
maximum |
|
Cost of reviews |
£1,044,761 |
£2,873,565 |
|
Net drug reduction |
£2,137,077 |
£8,509,982 |
|
|
||
|
Indirect benefits: avoidable bed days and admissions |
||
|
Definitely avoidable hospital bed days* |
362 |
|
|
Associated cost avoidance of definitely avoidable admissions |
£133,368 |
|
|
Possibly avoidable hospital bed days |
2,535 |
|
|
Associated cost avoidance of possibly avoidable admissions |
£933,576 |
|
|
* Including assumption that 10% of avoided bed days are avoided due to polypharmacy reviews |
||
|
|
Costs of reviews (£m) |
||
|
minimum |
maximum |
||
|
Net drug savings & indirect benefits* (£m) |
£1.04 |
£2.87 |
|
|
minimum |
£3.20 |
£2.16 |
£0.33 |
|
maximum |
£9.58 |
£8.53 |
£6.70 |
|
* indirect benefits of definitely avoidable admissions only |
|||
